Introduction to Batteries
by Jim Ewing
Great Hobbies Inc
Most of the batteries we use in our hobby today are the rechargeable type. There are several kinds of rechargeable batteries and these include NiCd (Nickel Cadmium), NiMH (Nickel Metal Hydride), Li-Po (Lithium Polymer), lead-acid, sealed lead-acid, and gel-cell, among others. NiCds are used to run our radio systems as well as power our model cars, boats, and planes. Generally they are wired together in packs of four or more cells, depending on the application. NiMH are relatively new and are being widely accepted for the same applications as NiCds. Li-Po cells are very new technology and are quickly finding their way into model applications. The other types of batteries mentioned are usually 6 or 12 volt and used to power flight boxes and large scale boats.
 NiCd Batteries
NiCd Batteries
Nickel Cadmium (NiCd) batteries are used in just about every radio system that comes with rechargeable batteries, and they are the main power source for most electric cars, boats, and many planes in the hobby. A NiCd cell, regardless of capacity, has a nominal voltage of 1.2V. When fully charged it will have slightly higher voltage and it is considered to be fully discharged when it is down to 1.1V.
The capacity of NiCds is measured in milliamp-hours (maH), the average current drawn times the time in hours. A NiCd cell of 1000maH capacity will ideally supply 1000ma of current for one hour, although efficiency is usually somewhat less (about 5 to 8% less). It will also ideally supply 2000ma of current for a half hour or 500ma of current for 2 hours (again, less about 5 to 8%). Delivered capacity is somewhat rate dependent. The faster you drain the cell the less mAH you can obtain to any given cut-off voltage. This is due to the internal resistance of the individual cells. This internal resistance will dissipate some of the power as heat and the amount dissipated has a “square” relationship to the current drawn. NiCds can be found with capacities ranging from 50maH to 4400maH in different size and shape packages.
Most radio systems have battery packs made up of AA size cells, having a capacity of 600maH. The airborne system battery pack will generally have 4 cells wired in series producing 4.8V and the transmitter will have 8 cells producing 9.6V nominal. Only the voltages add when the cells are wired in series; the capacity remains 600maH. In some applications it is desirable to have a greater capacity in the airborne system battery pack and even increase the number of cells by one to produce 6.0V. This gives greater torque and speed to your servos. It will also increase the current demand from the pack.
Battery packs to power car and truck models are usually made up of the Sub-C size cells. At one time these were rated at 1200maH in capacity, but with progress in technology, Sub-C’s are commonly found with 2400, 3000 and even 3300maH capacities. These packs are usually in either 6-cell (7.2V) or 7-cell (8.4V) configurations.
Pack sizes for aircraft vary more widely depending on the size of the model. Light indoor electrics use very small cells with as little as 110mAh capacity 4 or 5 cells per pack, while larger airplanes, may have 28 or more high capacity cells wired in series to form a pack.
Parallel operation of NiCds is a good way to get extra capacity. In doing so you must use packs with the same number of cells. The capacity of the packs may be different, however. While they may be discharged in parallel (the capacity of the two packs will be the sum of their individual capacities) you must have provision for charging them separately. Many modelers employ a dual set of switch harnesses that places the batteries in parallel for flight but separates them for individual charging. This significantly increases the system reliability since faulty switches and connectors account for far more “incidents” than faulty batteries (assuming one checks his packs occasionally).
When operating a radio control system it is very important to know the condition of the batteries powering it. The life of your model, and the safety of those around it, depends on this. Always be certain your transmitter and receiver battery packs are fully charged before you operate your model. Your transmitter will usually have a meter indicating the current state of your transmitter battery making it easy to monitor during operation. One way you can determine the state of your receiver battery is to plug an expanded scale voltmeter into your pack and measure the voltage under load. Doing this after each flight during a flying session is a good habit to acquire. Consider using an external charge jack on your model to more easily facilitate this.
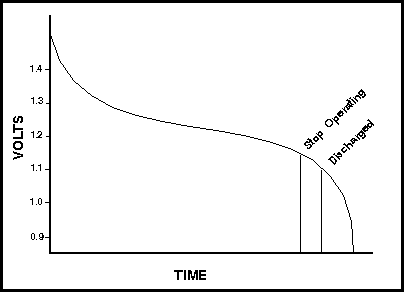
NiCd batteries discharge differently than do alkaline or other non-rechargeable types. When the cell first comes off charge it will usually show a fairly high voltage (1.4V possibly). This will drop off quickly as the cell is discharged until close to the nominal voltage of 1.2V. The voltage will then drop off slowly throughout the bulk of the discharge. However, once the cell is nearly fully discharged, it will drop off very quickly again. Refer to Figure 1 illustrating the cell voltage over the discharge time for a typical NiCd cell.
Although controversial, it is believed by many that NiCd batteries have one bad characteristic in that they can develop a memory. If a NiCd battery is repeatedly fully charged and then used an amount that is less than full capacity (let’s say you charge and regularly have three or four flights in a session), after a period of time, it may not be able to deliver any more than the capacity frequently used. This is called NiCd memory. Quite often this apparent memory condition may simply be voltage depression where the voltage of a cell is slightly less than rated. This can happen for several reasons such as operating at higher temperatures, extended overcharging, operation below 0°C, etc. The cell may actually be operating at or close to full capacity but the voltage appears slightly less. Memory occurs because the micro crystal structure of the cell becomes crystalline (small crystals join to form a larger unit) when not used, reducing the energy producing characteristics.
A way to avoid this “memory” is to cycle the batteries. Cycling is where the battery is fully discharged under controlled conditions (as described below) and then recharged. By fully discharging the pack in this way every so often, the cells in the pack will not develop memory and will remain at maximum possible capacity.
It is also a good idea to measure the capacity of your battery packs on a regular basis. This will tell you how long you can safely operate your model in one session. You will also discover when your batteries need to be replaced without destroying a good model in the process.
It is possible to check the capacity of the battery while cycling. If you discharge your pack at a constant, known rate, and measure the pack voltage at various time intervals during discharge, you can determine the capacity by multiplying the discharge current rate by the time it takes to fully discharge. Your pack is considered discharged when it reaches a value of from 1.0 to 1.1 volts per cell. For example, if you are discharging a 4-cell receiver pack, it would be fully discharged at between 4.0 and 4.4 volts (4 cells x 1.0 to 1.1 volts/cell). Do not discharge a pack below this level or cell reversal could result. Should a pack be discharged to a very low state by accidentally leaving the switch on, it should get a 24 hour charge with the system charger to bring all cells back into balance.
Battery cyclers are useful tools for both cycling the batteries and determining their capacity. They can do the battery maintenance automatically for you. You will find these electronic devices in the Great Hobbies catalog under “Electronics and Accessories”. It is definitely worth investing in one of these units as one crash due to poor battery maintenance will usually cost you more than a cycler, not to mention the hours you have put into building your model.
The normal charge rate for NiCds is C/10 or the capacity of the NiCd divided by 10. For example, a 600mAh pack should be charged at 600/10 or 60ma. This is known as the overnight rate. Although, ideally, a pack should be fully charged in 10 hours, due to inefficiency, it will probably take between 12 and 15 hours.
After being charged overnight, the battery should either be removed from the charger or the charge rate should be reduced to C/100 (the capacity divided by 100). This is known as the trickle rate. The 600mAh pack in our example would have a trickle rate of approximately 6ma. The battery may remain on the trickle rate indefinitely. Keeping your system’s batteries on trickle charge is a great idea as it will ensure that your batteries are fully charged when you go to the field. NiCds, just sitting around will probably loose 1% of their charge each day.
Most NiCds may also be charged at a higher rate such as a rapid charge of C (charge rate equal to capacity) or a quick charge of 4C or four times the capacity. This is normal practice with the packs used in powering electric models. Some cells are better at accepting a fast charge than others and these are usually denoted by being an “R” type cell or “SCR”. In fast charging NiCds, however, one has to be very careful to ensure that they do not get overcharged. Applying these high charge currents to a battery that is fully charged can at the least ruin the battery and at worst make the battery explode.
There are two basic types of NiCd fast chargers. The first is a timed charger where the charge rate is applied by turning on a timer. Timers are usually for 15 minutes although it will probably take between 20 and 25 minutes to fully charge the pack. Although timed chargers are not the best choice, they are often the most economical. Most timed chargers will come with a discharge circuit. The discharger is used to fully discharge the pack before charging so the current state of charge for the pack is known and to reduce the chance of overcharging it.
A second type of fast charger is the peak detection charger which can automatically charge your battery packs for you without the need for a timer. A circuit monitors the voltage of your pack during charge. As a NiCd charges, the voltage will increase at a slow rate. However, once the battery is fully charged, the voltage will “peak” and actually drop back slightly. The circuitry detects this drop and reduces the charge rate to trickle. You can safely charge your batteries with this type of charger and there is no need for initially discharging them.
Even though NiCds can be fast charged, it is important to slow charge your batteries at the overnight rate periodically, or about every 5 charges. This helps stabilize the cells to retain their full capacity and will lengthen their life.
Another important characteristic of NiCds that should be noted is their self discharge. Self discharging is the energy that the cell loses just sitting on the shelf with no load. Typically for NiCds, they will lose 10 to 15% of their capacity within 24 hours of coming off charge then reducing further by about 10 to 15% per month thereafter. Self-discharge rates increase with increased ambient temperature. It is a good idea to charge the batteries just before you go flying rather than charging them as soon as you get home and then not again before flying a few weeks later!
NiCd batteries are not environmentally friendly. They contain Cadmium which is dangerous to the environment. These batteries must be recycled and cannot be simply discarded in the waste.
 NiMH Batteries
NiMH Batteries
Nickel Metal Hydride (NiMH) batteries are very similar to NiCds, and have been accepted with open arms to the radio control community. A NiMH cell has the same nominal voltage of 1.2V as a NiCd so they can form packs in the same way. Where the NiMH cell really shines and has the advantage over the NiCd is in its capacity. For the same size package, the NiMH cell carries double the energy capacity. For example a typical ‘AA’ cell NiCd has a capacity of 700mAh whereas the NiMH ‘AA’ has about 1400mAh of capacity. The energy to weight ratio for NiMH is much better—almost double.
There are tradeoffs, however. The NiMH cell has a higher internal resistance (almost double that of the NiCd) and as a result is limited to the speed at which energy can be charged or discharged with the cell—lower charge rates and lower discharge rates. This is particularly true of the smaller NiMH cells such as ‘AAA’ and ‘AA’, however, some of the larger capacity cells (currently in the 3000 to 3300 mAH range) are designed for higher current rates. In some applications, such as powering electric model aircraft, the higher internal resistance can be noticeable on the power output when compared to NiCds. To help offset the drop, an extra cell is often added to the pack. For instance, where a 6-cell 7.2V NiCd pack is used, select a 7-cell 8.4V NiMH pack. Internal resistance increases with drop in temperature and decreases with increased temperature.
Another disadvantage to the NiMH is the useable working life. “Lifespan” in terms of age for NiCds and NiMH cells is similar, around 4 or 5 years, however, NiCds are capable of a greater number of usage cycles. Typically NiCds are capable of 1000 or more discharge/charge cycles whereas NiMH cells are limited to around 300 to 400. This may or may not be limiting for the user, depending upon one’s application.
Self-discharge also applies to NiMH cells. In fact, one may experience a higher self-discharge rate with NiMHs than with NiCds.
Although NiMH cells have been touted as not having “memory” like NiCds, this is not completely true. The nickel component in these cells is partially responsible for the memory effect. In a NiCd, both the nickel and the cadmium contribute to the memory while in the NiMH cell, the nickel is the only component contributing to the effect. As a result, NiCds show the effects of memory considerably more than NiMH. Cyclers can still play an important role in the exercising of NiMH to keep them delivering optimum capacity and output.
Charging characteristics for NiMH is slightly different than NiCd, particularly around the full charge point. Peak chargers used should be designed for NiMH cells and not just for NiCds to assure they turn off after peaking.
One other advantage for NiMH cells that should not be overlooked is that they are more environmentally friendly than NiCds—NiCds containing cadmium which is a chemical not desirable to have loose in the environment. NiMH may be simply discarded in the waste when they have reached the end of their life.
 Li-Po Batteries
Li-Po Batteries
Lithium Ion or Lithium Polymer batteries are quite new to the hobby, but they are showing early on that they promise great things, especially in the realm of electric indoor flight.
Unlike NiCd and NiMH cells the Li-Po cell has a nominal voltage of 3.6V. Typically these cells would be used in 1, 2, or 3-cell packs giving voltages of 3.6, 7.2, and 10.8 volts respectively. This limits the selection a bit when compared to the other batteries which offer combinations in 1.2V increments for wider voltage possibilities. The advantage comes in their energy density or energy to weight ratio—as much as four-fold over a typical NiCd battery! Modelers who have adapted these batteries for indoor and park flyers have found they can get long run times compared to using NiCd and NiMH packs.
Self-discharge for the Li-Po is very slight compared to either the NiCd or NiMH. It is generally negligible and one need not be concerned with topping up the cell before use.
Unlike NiCd and NiMH cells, the characteristics of the Li-Po cell remain relatively constant over many cycles. The internal resistance increases only marginally, the capacity decreases only marginally, and the self-discharge is still negligible. Unlike NiCd and NiMH cells, the Li-Po cell exhibits no “memory”. One can charge or discharge the pack partially or fully and still retain the long term capacity of the unit. This means that cycling with a battery exerciser is not required to maintain functionality—Li-Po are considered low maintenance as opposed to the NiCd and NiMH which are high-maintenance batteries.
So far, everything seems perfectly rosy and the Lithium cells are the cats-meow. There are some disadvantages, however, and also some cautions to take when using the Li-Po technology. As the technology improves—and it is changing quickly—these will undoubtedly become less of a concern.
The internal resistance for Li-Po is relatively high and thus the discharge rates will be lower than with the NiCd. Most discharge rates are in the range of 5C (five times the capacity) although some have recently been released that can supply 20C (for a 340mAh pack for instance, one could draw a maximum of 20 x 0.340 or 6.8A—still fairly low for some model power applications).
This internal resistance will also limit the charge current. As a matter of fact, to this point, charge rates should be restricted to 1C or equal to the capacity. That same 340mAh pack should only be charged at a maximum of 340ma , taking more than an hour (accounting for inefficiencies) to charge the pack. This may be a little longer than modelers are willing to wait in some circumstances after being used to charging one pack of NiCds while you fly another, then having the first ready to use again by the time you land.
While on the topic of charging, you cannot use the same type of charge circuitry as with the Nickel based cells. Li-Po cells must be charged with chargers designed specifically for them. Do not use chargers that are not designed for the purpose—serious and dangerous consequences could result.
Lithium cells can be very dangerous in a number of circumstances: being discharged too low; being overcharged (maximum voltage on a cell should not exceed 4.2V); being overheated. These batteries should never be charged unattended, nor should they be charged in an environment that is flammable in case the cell “flames-out” or worse . . .
Although cycling the Li-Po battery does not degrade it, age deteriorates it. The warmer the storage temperature the faster the aging occurs. At room temperature, you can expect a lithium cell to last only between 1 and two years at best. At warmer temperatures you would be lucky to get one year useful life. Once these cells have reached the end of their life, simply puncture the plastic envelope surrounding the pack and submerse the pack in salt water for a few hours. You can then place it in the waste.
Lithium batteries are still in their infancy and as a result the technology is improving at a rapid rate. Certainly it will advance a great deal throughout the life of this catalog. Keep an eye on its progress and see where Li-Po cells can fit into your modeling future.
 Field Box Batteries
Field Box Batteries
The other type of batteries such as lead-acid, sealed and non-sealed, and gel cells should also be charged with care. DO NOT charge your field box battery with an automotive car charger or with any other “fast charger”. These batteries can boil dry and be damaged by high rates of charge. Use a charger meant for the job at the overnight rate of C/10.
Resources
There are many great resources available for learning more about batteries and opinions can vary. Since the internet is one of the mostly widely available tools for reference, we will provide you with sources for further reading. These sources also provided inspiration for much of the material presented here.
- Isidor Buchmann, Batteries in a Portable World: http://www.buchmann.ca/
- Fred Marks, FMA, Inc: https://www.fmadirect.com/
- Red Scholefield’s RC Battery Clinic: http://www.rcbatteryclinic.com

